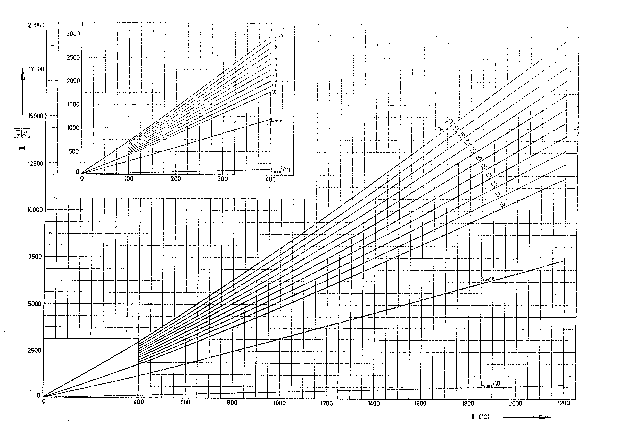
Minimal quantity of air:
A boiler operates under the following conditions:
Steam production: D=70 kg/s
Steam pressure: p1=120 b
Steam temperature: ts=350 °
C
Feed water temperature: ta=200 °
C
Preheated air temperature: tL=250 °
C
a) Estimate a boiler ballance if given are the excess air l= 1,25 and boiler efficiency coefficient hk = 0,80. Fuel is given: composition, c=0.195; h=0.017; s=0.008; o=0,10; n=0,004; w=0,470; a= 0,198 and lower calorific value Hd=5930 kJ/kg. Furnace efficiency ishF=0,94;hg=0,98 and insulation hz=0,98.
b) What happens if the air preheater is evenly split into two parts positioning a water heater between them.
c) Estimate a reversibility coefficient of the boiler.
Solution:
a) Refer Example 4 for more details and plot H-t diagram:

Minimal quantity of air:
Air necessary for the combustion:
VL =l VLmin = 2,317 (Nm3 /kg)Minimal quantity of combustion products:
VRWmin = 1,867c+11,2h+ 0,7s+1,244w+0,79 V Lmin + 0,8n = 2, 617 (Nm3 /kg)Real quantity of combustion products for a given l:
VRW = VRWmin+ (l -1) VLmin =3,081(Nm3 /kg)Heat Ballance
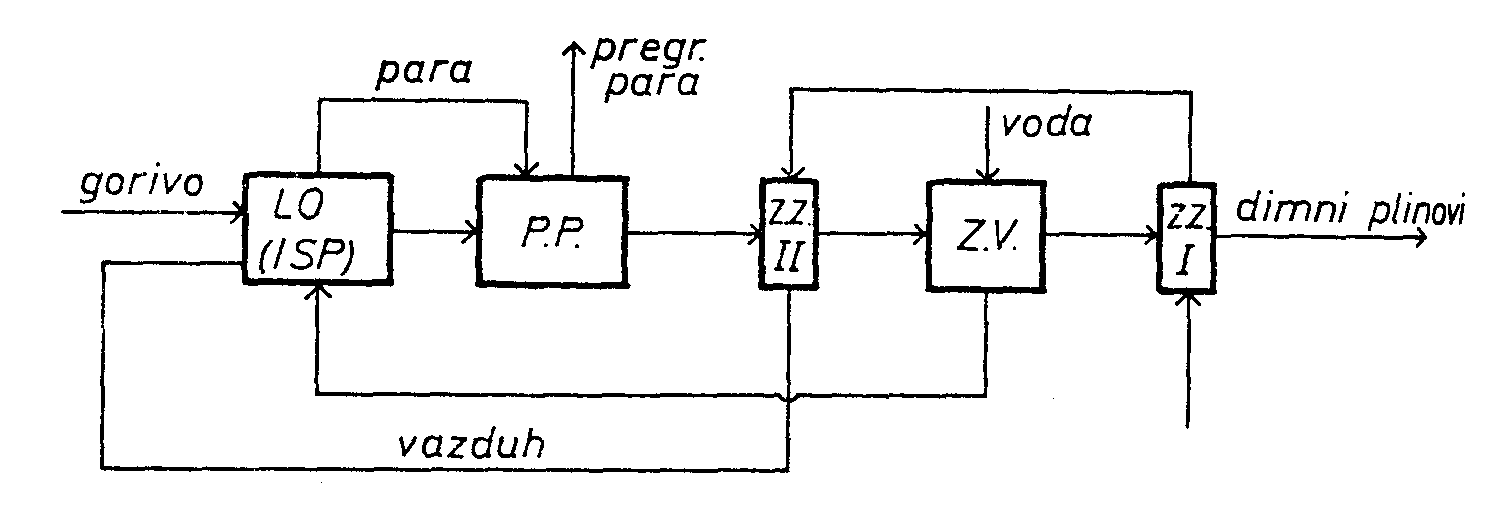
The boiler is given on the diagram:
LO - Furnace and evaporator
PP - Superheater
ZV - Water heater
ZZ - Air preheater
![]()
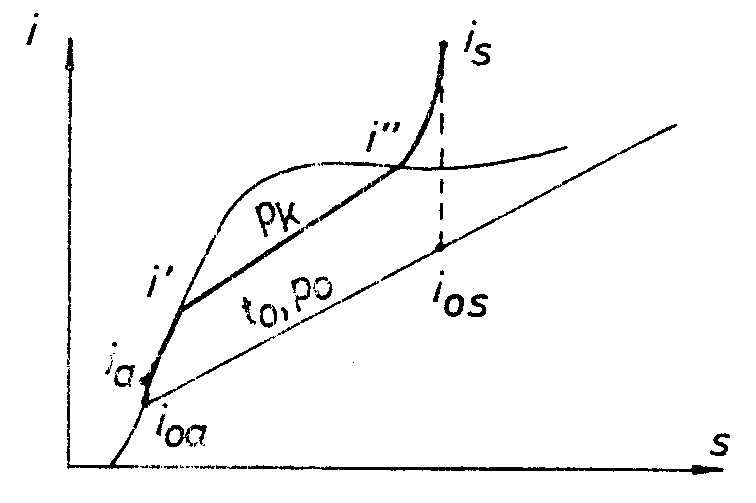
Heat exchanged
Heat exchanged in the water heater:
QE = D (i ’-ia ) = 70 (1490 – 898) = 41400 (kW)Heat exchanged in the evaporator:
Qi = D (i” – i’) = 70 (2685 – 1490) = 83650 (kW)Heat exchanged in the the superheater:
Qs = D (is - i”) = 70 (3420 – 2685) = 51450 (kW)Quantity of fuel needed for combustion:
Heat exchanged in the in the air preheater: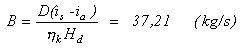
QZ = hz B(iL –il)VLiL - specific enthalpy of the preheated air
for tL= 250° C, iL= 335 (kJ/Nm3) and for tl = 20 ° C , il = 26 (kJ/Nm3), therefore:
Qz = 26640 (kW)Temperatura di stribution in the boiler:
Theoretical temperature in the furnace is obtained from the HI-t diagram based on the theoretical enthalpy HFo and excess of air:
IFo =Hd hF + VL iL = 6000 ( kJ/kg) , l = l,25and from H-t dijagrama it is:
t Fo = 1280 ° C.Temperature after the furnace (evaporator) is obtained from the corresponding enthalpy when a heat exchanged in the evaporator is subtracted from the theoretical enthalpy:
This enthalpy and excess of airl = 1,25 give from H-t diagram:
tF2=800 ° C.Enthalpy of combustion products after the superheater is:
and a corresponding temperature:
tF3 = 500° C.Enthalpy of the combustion products after the water heater is:
for which accompanied with excess of air the temerature from H-t diagram is:
tF4 = 300 ° CFinaly, enthalpy at end of the boiler is:
Temperature of the combustion products at the boiler end is obtained from the H-t diagram as:
tF5 = 160 ° C.Lenz t-Q diagram for this boiler is shown in the figure:
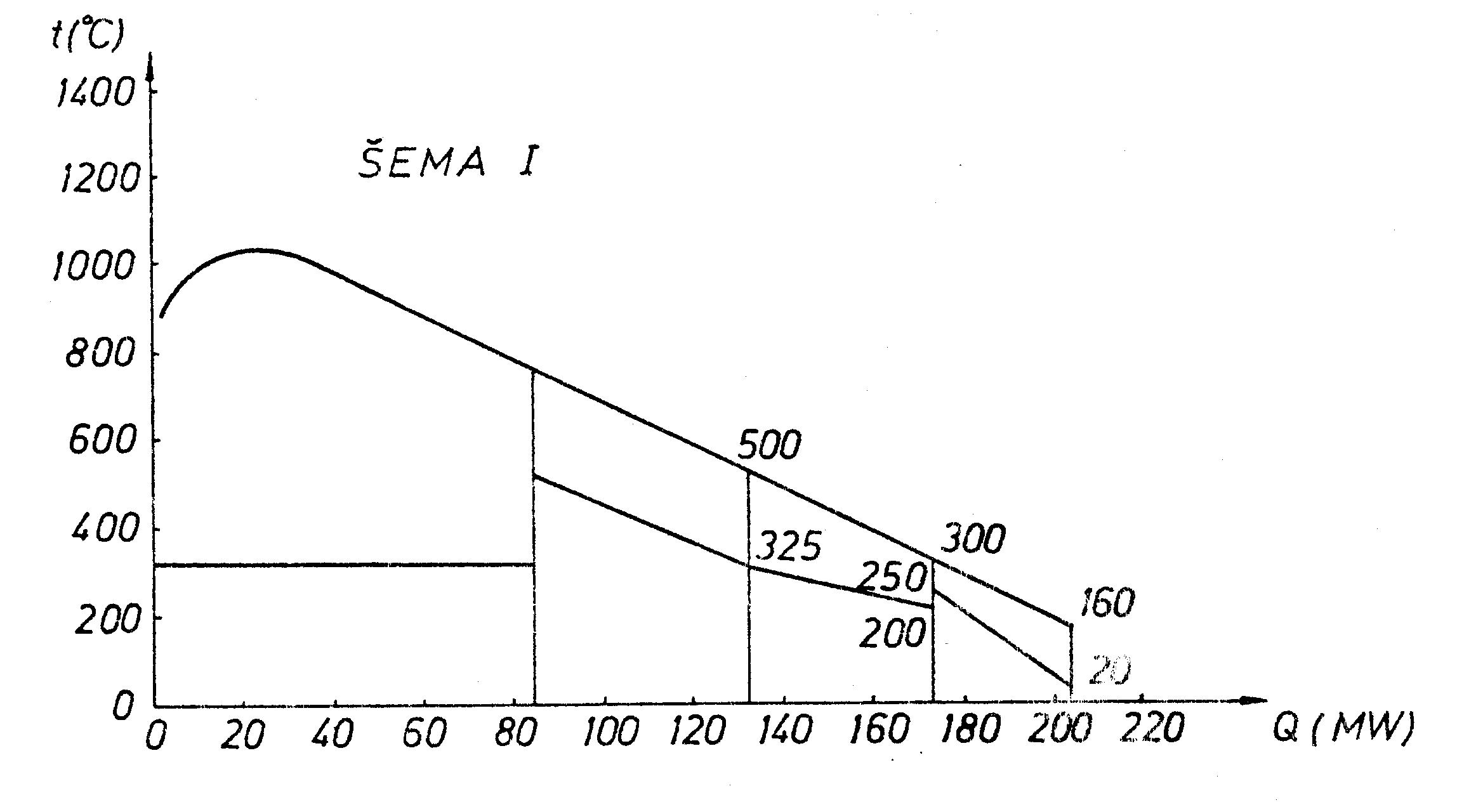
b ) If at the air preheter is split into two equal parts and a water heater is placed between them, the boiler becomes:
Heat exchanged in each part of the air preheater:
LO - Furnace and evaporator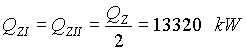
Temperature of combustion products at the end of the second preheater stage is obtained for the H-t diagram:
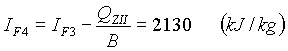
TF3 = 466 oC.
Enthalpy of combustion products
at the water heater exit is:
and a correspoonding temperature:
t F5=215 oCEnthalpy at the boiler exit is:
which is identical to the previous case which is the same for the exit temperatures.
Lenz diagram is for this case:
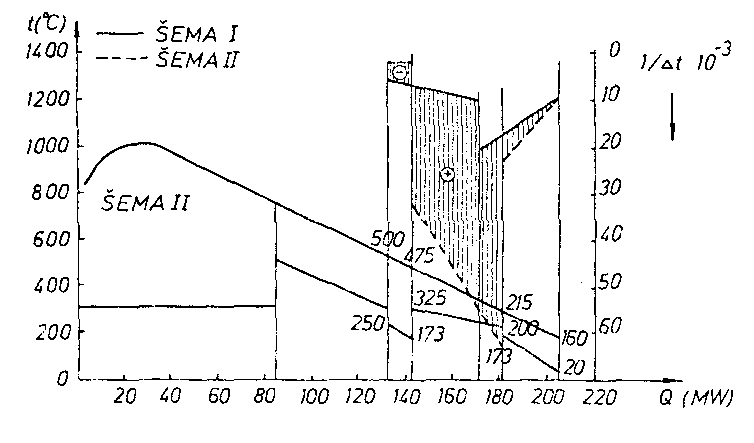
To compare two boilers a distribution of the reciprocals of temperature
differences is plotted representing the values of
kA. For the same koefficient of heat transfer, the boiler I requires a
smaller heating surface than boiler II.